bioNexia® FOBplus
Early detection of colorectal cancer
The bioNexia® FOBplus rapid test is a non-invasive method for colorectal cancer screening.
- Very sensitive Fecal Occult Blood test
- Results available at just 5 minutes
- User-friendly method for increased patient comfort and easy interpretation
Colorectal cancer is one of the most common causes of death from cancer. However, if it is detected at a very early stage, there is an over 90% chance of survival. Adenomatous polyps, that have the potential to transform into cancer, tend to bleed frequently. It is known that their early removal reduces the risk of colorectal cancer1.
The bioNexia® FOBplus rapid test allows the detection in stools of human hemoglobin potentially linked to adenomatous polyps. Without automatically pointing to cancer, a positive test result indicates a disorder which should be investigated further.
A solution to fit your needs

bioNexia® FOBplus – different kits for different needs (Click to enlarge)
High sensitivity
The bioNexia® FOBplus test is much more sensitive than Guaiac-based tests2 in correlation with clinical diagnosis.
Diagnostic sensitivity
| bioNexia® FOBplus | Guaiac-based FOBT | |||
|---|---|---|---|---|
| Clinical Diagnosis | Positive results | Sensitivity % (95%CI*) |
Positive results | Sensitivity % (95%CI*) |
| Advanced adenoma | 68/130 | 52.3 (43.4-61.1) | 12/128 | 9.4 (4.9-15.8) |
| Other adenoma | 77/275 | 28.0 (22.8-33.7) | 9/260 | 3.5 (1.6-6.5) |
| Any adenoma | 145/405 | 35.8 (31.1-40.7) | 21/388 | 5.4 (3.4-8.2) |
*Confidence interval
The performance characteristics were assessed on a target population for screening. The sensitivity of the bioNexia® FOBplus test for the detection of all adenomas is 35.8%, and for the Guaiac-based FOBT it is 5.4%
Diagnostic specificity
| bioNexia® FOBplus | Guaiac-based FOBT | |||
|---|---|---|---|---|
| Clinical diagnosis | Negative results | Specificity % (95%CI*) |
Negative results | Specificity % (95%CI*) |
| None or hyperplastic polyp | 749/914 | 81.9 (79.3-84.4) |
851/887 | 95.9 (94.4-97.1) |
The specificity of the bioNexia® FOBplus test is 81.9% for patients with a negative colonoscopy.
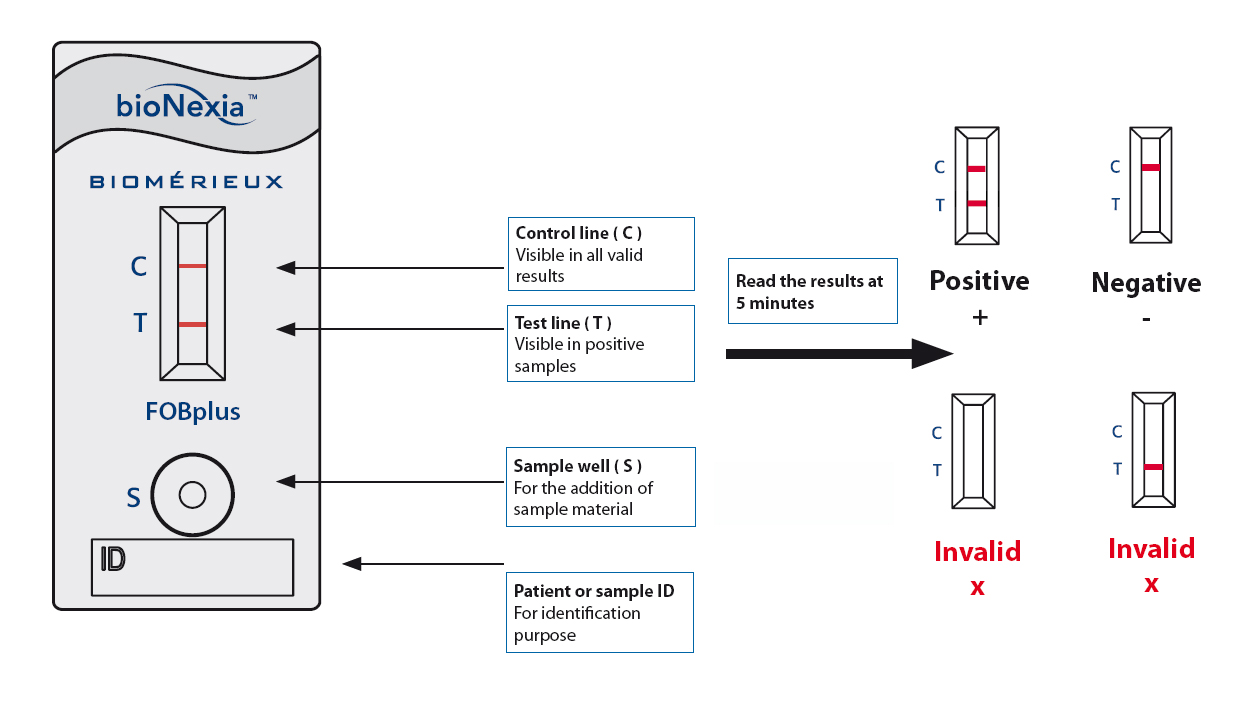
Easy-to-read results
Click to enlarge
2 red bands = positive*; 1 red control band = negative.
*A positive FOB test should always be followed-up by further investigations, such as colonoscopy.
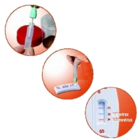
Increased patient comfort
- Patient set: biodegradable stool collection paper, sample tube
- Simple, “no mess” collection kit with clear instructions for use
- Single stool collection
- No special diet required before testing
- No reaction with non-steroidal anti-inflammatory drugs
References:
1. Atkin WS, Edwards R, Kralj-Hans I, et al. Lancet 2010;375(9726):1624-33.
2. Hundt S et al. Comparative Evaluation of Immunochemical Fecal Occult Blood Tests for Colorectal Adenoma Detection. Ann Intern Med. 2009; 150:162-169.
| bioNexia® FOBplus / All-inclusive kit | |
|---|---|
| Reference | 410594 |
| Contents | - 25 test cassettes - 25 patient sets, including stool paper, sample tube and instructions |
| Sample type | Stools |
| Analytical sensitivity | 40 ng/ml |
| Time to result | 5 minutes |
| Sample volume | 4 drops of sample extract |
| Storage temperature | + 2 to + 30°C |
| Shelf life | 24 months from date of manufacturing |
| bioNexia® FOBplus LK / Lab Kit | |
|---|---|
| Reference | 414275 |
| Contents | -25 test cassettes -25 sample tubes |
| Sample type | Stools |
| Analytical sensitivity | 40 ng/ml |
| Time to result | 5 minutes |
| Sample volume | 4 drops of sample extract |
| Storage temperature | + 2 to + 30°C |
| Shelf life | 24 months from date of manufacturing |
| bioNexia® FOBplus PS / Patient Set | |
|---|---|
| Reference | 414276 |
| Contents | 25 patient sets, including stool paper, sample tube and instructions |
Consult your local bioMérieux representative for product availability in your country.
Related Publications
Guidelines
European guidelines for quality assurance in colorectal cancer screening and diagnosis